Introduction: CARTITUDE-4 (NCT04181827) is a randomized, phase 3 trial comparing ciltacabtagene autoleucel (cilta-cel), a BCMA-directed CAR-T cell therapy, with standard of care (SOC; pomalidomide, bortezomib, and dexamethasone [PVd] or daratumumab, pomalidomide, and dexamethasone [DPd]) in patients (pts) with lenalidomide (len)-refractory multiple myeloma (MM). The trial recently showed that pts in the cilta-cel arm experienced significantly improved progression-free survival (PFS) vs SOC (HR, 0.26; P<0.0001) and a significantly higher rate of complete response (CR) or better and overall response rate (ORR) in the intent-to-treat (ITT) set (San-Miguel et al, New Engl J Med 2023). We report efficacy and safety in pts who received cilta-cel as study treatment (‘as-treated’ set) in the experimental (cilta-cel) arm of the study.
Methods: Pts had 1-3 prior lines of therapy (LOT), including a proteasome inhibitor and immunomodulatory drug. A single cilta-cel infusion (target dose, 0.75×10 6 CAR+ viable T cells/kg) was administered after apheresis, bridging therapy (DPd [28-day cycles] or PVd [21-day cycles]), and lymphodepletion. Treatment responses and disease progression were assessed per IMWG criteria; minimal residual disease (MRD) negativity at 10 -5 was assessed by next-generation sequencing starting at day 56 post infusion; time-to-event endpoints were analyzed using the Kaplan-Meier method. Cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity (ICANS) were graded per ASTCT criteria; other adverse events (AEs) were graded per NCI-CTCAE criteria.
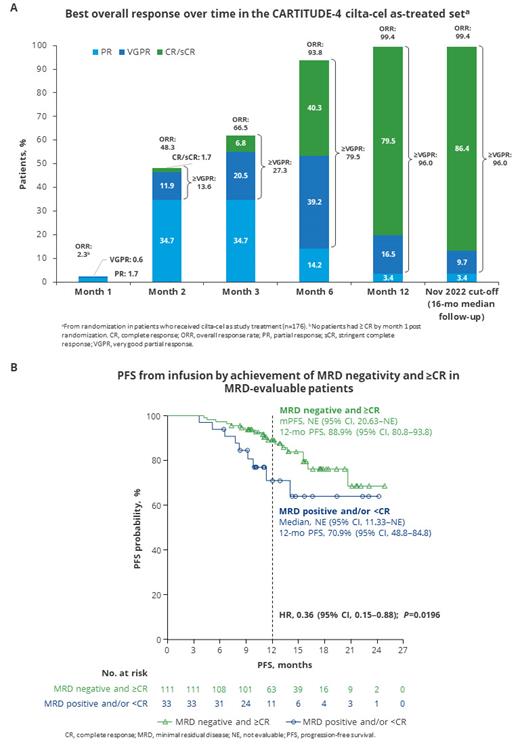
Results: As of Nov 1, 2022, 176 of 208 pts randomized to the cilta-cel arm had received cilta-cel as study treatment (median age, 61 years; 34% with 1 prior LOT; 6% ISS stage III; 60% with high-risk cytogenetic profile; 17% with soft tissue plasmacytoma; 13% with high tumor burden; 11% triple-class refractory). Baseline characteristics were consistent with those of the ITT set. In the as-treated set, median follow-up from randomization was 16 months (mo), and 22% of pts had received 1 bridging therapy cycle, 59% 2 cycles, and 18% 3 cycles. Disease burden was effectively controlled in the as-treated set during bridging therapy. Median PFS was not reached and the 12-mo PFS rate from infusion was 85%; 12-mo overall survival rate from infusion was 92%. 175 of 176 pts who received cilta-cel as study treatment responded (99% ORR), including 86% who achieved ≥CR. Median time from randomization to first response was 2.1 mo and responses deepened over time; 2% of pts achieved ≥CR by 2 mo post randomization, and rates increased to 40% by 6 mo and 80% by 12 mo (Figure A). Median duration of response was not reached. 72% of the as-treated set (88% of 144 MRD evaluable) achieved MRD negativity at any time. 111 of 144 MRD-evaluable pts (77%) achieved both MRD negativity and ≥CR. These pts had improved PFS vs pts who remained MRD positive and/or had <CR (HR, 0.36; 95% CI, 0.15-0.88; P=0.0196); respective 12-mo PFS rates were 89% and 71% (Figure B). The most common CAR-T cell-related toxicity was CRS (76% any grade [gr]; 1% gr 3). The overall CAR-T cell neurotoxicity rate was 21% (3% gr 3/4). ICANS occurred in 5% of pts (no gr 3/4). Other neurotoxicities occurred in 17% of pts (2% gr 3/4); these included cranial nerve palsy (CNP) in 9% (1% gr 3/4), peripheral neuropathy in 3% (1% gr 3/4), and 1 pt with gr 1 movement/neurocognitive treatment-emergent AEs (MNT). By clinical cut-off, CRS and ICANS had resolved in all pts; CNP and peripheral neuropathy had resolved in all but 2 pts; and the 1 MNT case had not yet resolved.
Conclusions: This analysis of the as-treated set in CARTITUDE-4 shows potent effects of cilta-cel in pts with len-refractory MM after 1-3 LOT. The PFS rate of 85% at 12 mo post infusion (89% in pts with MRD-negative ≥CR) compares favorably with real-world data in similar populations (12-mo PFS rate <50%; Lecat et al, Front Oncol 2021). Effective bridging therapy during the CAR-T manufacturing period may help ensure that pts can receive cilta-cel. In the context of longer-term data from cohort A of the CARTITUDE-2 study (Hillengass et al, ASH 2023, submitted) in a similar pt population, these results support the potential for prolonged disease control and the benefit of cilta-cel for pts with MM as early as after first relapse.
Disclosures
Sidiqi:Antengene, Janssen, BMS, Gilead: Speakers Bureau; Janssen, Pfizer: Membership on an entity's Board of Directors or advisory committees. Corradini:Celgene: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Amgen: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; ADC Theraputics (DSMB): Other: Honoraria (Consulting, advisory role, or lecturer); AbbVie: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Daiichi Sankyo: Other: Honoraria (Consulting, advisory role, or lecturer); Novartis: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Janssen: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Nerviano Medical Science: Other: Honoraria (Consulting, advisory role, or lecturer); Kyowa Kirin: Other: Honoraria (Consulting, advisory role, or lecturer); Incyte: Other: Honoraria (Consulting, advisory role, or lecturer); Gilead/Kite: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Sanofi: Other: Honoraria (Consulting, advisory role, or lecturer); Pfizer: Other: Honoraria (Consulting, advisory role, or lecturer); Roche: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; SOBI: Other: Honoraria (Consulting, advisory role, or lecturer); Takeda: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; GlaxoSmithKline: Other: Honoraria (Consulting, advisory role, or lecturer); BeiGene: Honoraria; Bristol Myers Squibb: Other: Travel and accomodations. Purtill:Gilead, BMS Celgene, Jazz: Honoraria. Einsele:Janssen: Honoraria, Other: Consulting or advisory role, Travel support, Research Funding; Sanofi: Honoraria, Other: Consulting or advisory role, Travel support, Research Funding; GlaxoSmithKline: Honoraria, Other: Consulting or advisory role, Travel support, Research Funding; Takeda: Honoraria, Other: Consulting or advisory role, Travel support; Amgen: Honoraria, Other: Consulting or advisory role, Travel support, Research Funding; Novartis: Honoraria, Other: Consulting or advisory role, Travel support; Bristol Myers Squibb/Celgene: Honoraria, Other: Consulting or advisory role, Travel support, Research Funding. Dhakal:Janssen, Karyopharm, GSK, Arcellx, GSK, Sanofi, Genentech, Pfizer: Consultancy, Honoraria, Speakers Bureau. Karlin:Amgen, Celgene, GSK, Janssen, Takeda: Consultancy; AbbVie, Amgen, Celgene, Janssen, Sanofi, Takeda: Honoraria. Manier:Abbvie, Amgen, Celgene/BMS, GlaxoSmithKline, Janssen, Novartis, Pfizer, Regeneron, Roche, Sanofi, Takeda: Membership on an entity's Board of Directors or advisory committees. Iida:Takeda: Consultancy, Honoraria, Research Funding; Bristol-Myers Scuibb: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria, Research Funding; Ono: Honoraria, Research Funding; Abbvie: Consultancy, Research Funding; Chugai: Research Funding; Novartis: Consultancy, Research Funding; Otsuka: Research Funding; Amgen: Research Funding; GlaxoSmithKlein: Consultancy, Research Funding; Alexion: Research Funding; Pfizer: Consultancy, Research Funding; Shionogi: Research Funding; Otsuka: Consultancy; Regeneron: Consultancy; Daiichi Sankyo: Research Funding; Janssen: Consultancy, Honoraria, Research Funding. Giebel:Pfizer: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; Gilead: Consultancy, Honoraria, Speakers Bureau; Abbvie: Consultancy, Honoraria, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Speakers Bureau; Amgen: Consultancy, Honoraria, Speakers Bureau; Roche: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Servier: Honoraria, Speakers Bureau; Swixx: Honoraria, Speakers Bureau; Angelini: Honoraria, Speakers Bureau; BMS: Honoraria, Speakers Bureau; Zentiva: Consultancy, Honoraria. Harrison:Haemalogix: Membership on an entity's Board of Directors or advisory committees; Abbvie, Amgen, Celgene/BMS, GSK, Janssen Cilag, Novartis, F. Hoffmann-La Roche Ltd / Genentech, Inc., Eusa: Speakers Bureau; Abbvie, Amgen, Celgene/BMS, GSK, Janssen Cilag, Novartis, F. Hoffmann-La Roche Ltd / Genentech, Inc., Haemalogix, Eusa, Terumo BCT: Honoraria; Celgene/BMS, GSK, Janssen Cilag, Haemalogix: Research Funding; Abbvie, Amgen, Celgene/BMS, GSK, Janssen Cilag, Novartis, F. Hoffmann-La Roche Ltd / Genentech, Inc., Haemalogix, Eusa, Terumo BCT: Consultancy. Lipe:Janssen, GSK, BMS, ABBVIE: Consultancy. Khan:Janssen: Honoraria; Secura Bio: Consultancy, Research Funding; BMS: Research Funding; Amgen, Sanofi: Speakers Bureau. Schecter:Janssen: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties: Janssen. Jackson:Janssen R&D: Current Employment, Current equity holder in publicly-traded company. Yeh:Janssen R&D: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Banerjee:Janssen: Current Employment, Current holder of stock options in a privately-held company. Deraedt:Janssen R&D: Current Employment. Lendvai:Janssen R&D: Current Employment, Current holder of stock options in a privately-held company. Lonardi:Janssen R&D: Current Employment. Slaughter:Janssen: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Li:Janssen R&D: Current Employment, Current equity holder in publicly-traded company. Roccia:Janssen: Current Employment, Current equity holder in private company. Patel:Legend Biotech, GSK, Freeline, BMS (spouse), AbbVie (spouse): Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months, Ended employment in the past 24 months. Florendo:Legend Biotech: Current Employment, Current equity holder in publicly-traded company. Koneru:Legend Biotech: Current Employment. Costa Filho:Legend Biotech: Current Employment, Current equity holder in publicly-traded company. Geng:Legend Biotech: Current Employment. San Miguel:Novartis: Consultancy, Honoraria; MSD: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Haemalogix: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; SecuraBio: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Regeneron: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal